Abstract
Approximately 30% of Acute Myeloid Leukemia (AML) patients are characterized by an Internal Tandem Duplication (ITD) in the FLT3 gene that is associated with a worse clinical course, a higher relapse rate, and shorter overall survival. The prognostic significance of measurable residual disease (MRD) positivity after chemotherapy or before hematopoietic stem cell transplantation (HSCT) has been largely demonstrated. Despite this, FLT3-ITD is not used as a marker of MRD because it is reported to be an unstable marker. Moreover, the methods currently used for the detection of FLT3 ITD are characterized by a low Limit of Detection (LoD), therefore small clones (<1%) cannot be identified. For this reason, it is difficult to monitor the persistence or reappearance of a FLT3-positive clone during remission and after HSCT. The possibility of identifying the FLT3-ITD clones as long as they are still very small would allow an early treatment with selective inhibitors.
This study aimed 1) to follow up small clones in patients with FLT3 ITD positive at diagnosis and negative after therapy. 2) to verify the presence of small clones at diagnosis in patients who were negative for FLT3-ITD at diagnosis and relapsed as FLT3-ITD positive.
We set up a patient-specific approach based on droplet digital PCR (ddPCR). We analyzed 48 samples from 11 AML patients. Seven patients were diagnosed as negative for FLT3-ITD but relapsed with a positive clone (group A), while 4 were identified as FLT3-ITD positive at diagnosis (group B). For each patient, the FLT3-ITD sequence was identified by Sanger sequencing and different patient-specific probes were designed. The LoD of each assay was calculated by mixing the DNA of a wild-type sample with an increasing amount of mutated DNA. Finally, the presence of FLT3-ITD clones was tested in the collected samples by a 2-plex ddPCR, having labeled the patient-specific probes with FAM and the total FLT3 probe with HEX. The target concentration in each sample was expressed as a percentage of FLT3-ITD/FLT3. ddPCR results were compared with the data obtained in the diagnostic routine with LeukoStrat CDx FLT3 mutation assay (Invivoscribe).
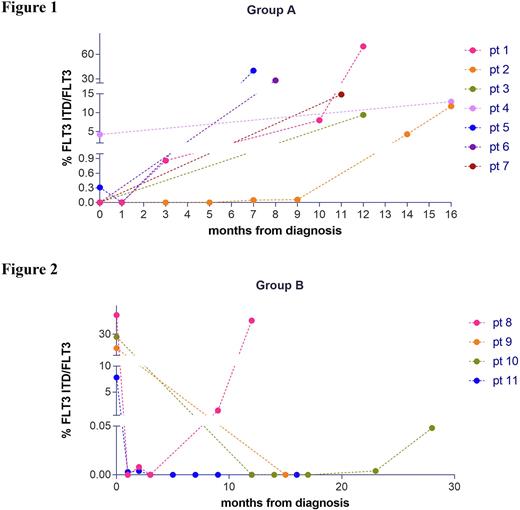
FLT3-ITD was evaluated at diagnosis and during follow-up in both groups. Regarding group A, ddPCR was able to identify 3 out of 7 patients, evaluated as FLT3 wild-type by the diagnostic test, in which the FLT3-ITD clone was already present at diagnosis, with a percentage of FLT3-ITD/FLT3 of 0.003%, 4.25% and 0.31% for patients 3, 4 and 5. (Fig. 1). In the remaining 4 patients, the absence of an ITD clone at diagnosis was confirmed by ddPCR, but we were able to detect the appearance of a small clone about 9 months before the relapse in 2 of them (pt 1 and 2). In Group B (patients FLT3-ITD positive at diagnosis by routine tests) several time points of follow up were evaluated by ddPCR (Fig.2). In 2 out of 4 patients, FLT3-ITD was negative after treatment and remained negative throughout the follow-up. In patient 8 ddPCR approach was able to identify the presence of a small mutated FLT3-ITD clone (1.43%), which was undetectable by routine test 9 months after diagnosis and 3 months before relapse. In patient 10 ddPCR detected the presence of a small FLT3-ITD clones of 0.004% 0.048% 8 and 3 months before relapse respectively. Our study demonstrates that in FLT3-ITD negative patients a very small clone can be present at diagnosis or can appear soon after the end of chemotherapy. In both cases this clone remains under the limit of detection of 5% for months until the overt relapse. These data are purely cognitive at the moment because the probes are designed on the ITD at relapse, but these data provide the rational for the development of a "universal" ddPCR able to detect the majority of ITD sequences. Finally, our study demonstrates that in patients FLT3-ITD positive at diagnosis, ddPCR allows to detect the persistence or the reappearance of small clones that expand until clinical relapse. The possibility of an early diagnosis of molecular relapse offers a window of time for intervention with selective FLT3 inhibitors in the attempt to prevent or delay relapse.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal